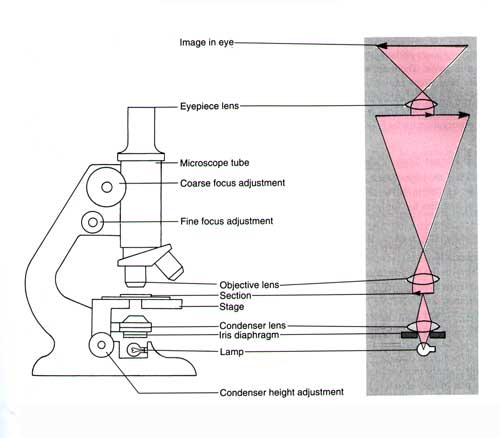
Some Drawbacks to Light Microscopy:
1. The resolving power is limited:
It is important to know understand what the resolving power (resolution) of a light microscope is.
Resolution = 0.61 x λ /NA
where λ is the wavelength of the illuminating radiation, and NA the numerical aperture of the lens.
For a light microscope, the highest practicable NA is around 1.4. For white light (lambda is approximately 0.53
What this means is that, under optimal conditions, with a high numerical aperture lens, you can only resolve, or see as separate particles, two particles that are more than 200 nm apart.
2. The specimen is dead.
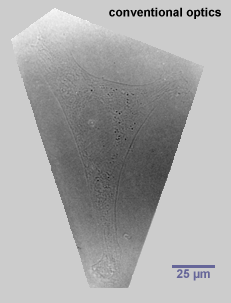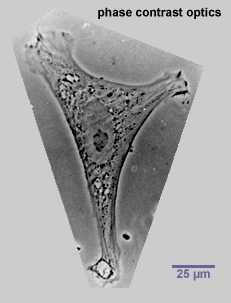
When cells are fixed and stained, the fixing procedure kills the cells. It is hard to know what the cells were involved in, from the dead remains, or what they might have been doing prior to, or gone on to do after, fixation. A technique that gets around this problem to some extent is a specialised form of microscopy called phase contrast microscopy, which generates black and white images of transparent cells by exploiting the different refractive indices of the various components within them. Even using this technique it is rarely possible to study the behaviour of cells in intact tissues because they are hidden from view, but the morphology and activities of living cells can be observed if they are removed from the body and kept alive in tissue culture.